Today’s blog will focus on Narcan, a critical opioid overdose rescue drug that is produced from thebaine, Antheia’s first commercial-scale, biomanufactured pharmaceutical ingredient. Read on for more on recent efforts to make Narcan available over the counter, as well as the challenges and opportunities this policy change presents for the pharma industry.
After the U.S. saw a devastating increase in opioid-related deaths starting in the 2000s, both the public and private sectors took action to address the crisis. In addition to law enforcement efforts targeted at illicit markets, consumer education campaigns, and changes in prescription practices, naloxone – an essential medicine for reversing opioid overdoses – became a critical part of the solution.
Advocates pushed for regulators to make naloxone more readily available, both for people struggling with addiction and for families, bystanders, and community groups working to help them. Naloxone was previously only available with a prescription for intravenous or intramuscular use and was mainly reserved for overdose treatment in a hospital setting. In 2023, with support from advocacy groups, the U.S. Food and Drug Administration (FDA) approved Narcan, a brand name version of naloxone, as an over-the-counter (OTC) medication.
Narcan is now available as an OTC nasal spray, with user-friendly instructions on how to administer it, making it available to the public for use anywhere that an overdose can occur – not just at hospitals. The FDA’s vision for increasing access includes having Narcan in stock at drug stores, convenience stores, grocery stores, gas stations, and online.
This is a positive move that will undoubtedly save lives, but the pharma industry’s long, complex, and fragile supply chains are unprepared to adapt to this growth in demand.
Traditional Narcan production
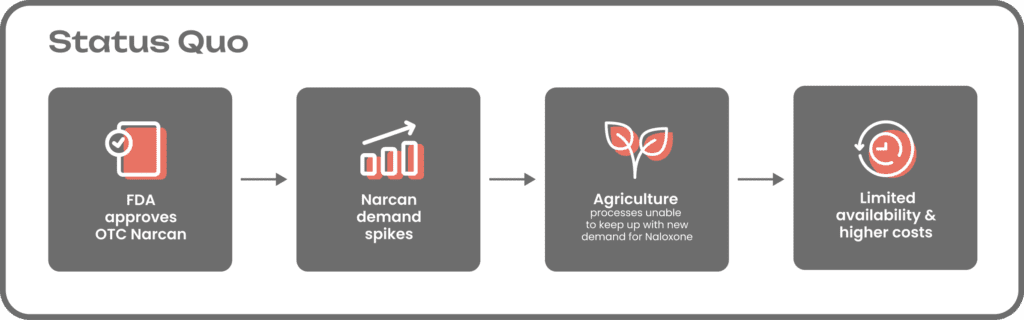
The active ingredient in Narcan is naloxone, a derivative of the key starting material (KSM) thebaine, which traditionally is sourced directly from the poppy plant.
Growing poppy plants takes years, and the process is vulnerable to many variables, including climate change, farming practices, soil health, and pests and disease. Any issues that arise during the cultivation process can have a significant impact on yields, making it nearly impossible for growers to respond to sudden changes in demand.
Once the mature poppy plants are harvested, cultivators extract thebaine and ship the compounds to active pharmaceutical ingredient (API) manufacturing centers. Upon arrival, thebaine undergoes a complex, seven-step chemical synthesis process to convert it into naloxone and then moves through the rest of the global pharma supply chain, which comes with its own unique set of challenges and vulnerabilities.
Further compounding the problem, only a limited number of farmers in a handful of foreign countries are permitted to legally grow poppy plants. In other words, there is no U.S.-based supply of thebaine or other critical poppy-sourced pharmaceutical ingredients that are used in the formulation of more than half a dozen critical medicines, beyond just Narcan. The U.S.’ lack of domestic supply is a national security risk as geopolitical alliances around the world can and will continue to shift.
Challenges of ramping up production and adoption
The current Narcan manufacturing process can take years, which means asking drugmakers to quickly increase production to meet OTC demand is much easier said than done. Existing facilities are only designed for legacy manufacturing technologies and historical levels of demand, so producers may need to invest in infrastructure upgrades. This process is well underway, but the public rollout of OTC Narcan has been uneven in the months since the FDA’s decision, with availability varying widely across states.
Recent federal data showed opioid-related deaths have dropped roughly 10.6% in the last year, likely due to a mix of factors, including wider availability of Narcan. In 2023, 22 million doses of Narcan were distributed in the U.S. and Canada. Though that 10.6% drop is significant, there is still a long way to go to get Narcan into more hands and save lives.
Another factor impacting Narcan adoption by the public is the cost. Right now, OTC Narcan is sold for $45-50 for a two-dose pack. This price is out of reach for many people impacted by addiction, adding another barrier to access. Lengthy and unpredictable agricultural cultivation processes make Narcan manufacturing more expensive for producers, which can impact the costs for consumers.
How biomanufacturing can address production issues to meet demand
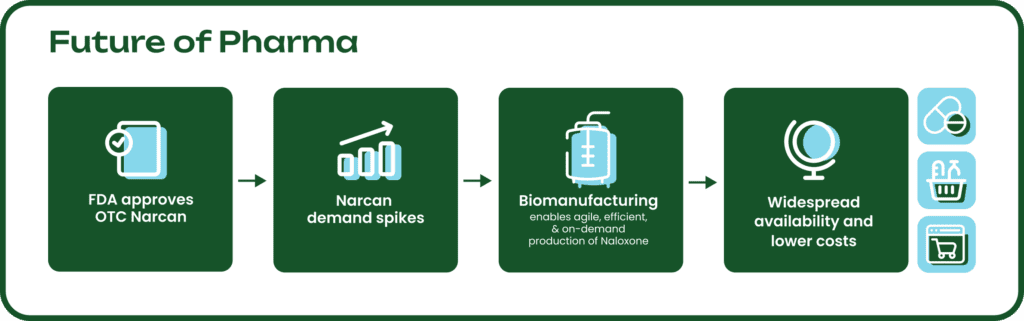
To boost Narcan production, manufacturers need reliable access to its source material, thebaine. Newer technologies like biomanufacturing can significantly speed up the process and help meet the surge in demand. Importantly, there is also a rise in new products formulated with naloxone for the prevention and treatment of addiction, not just overdose reversal. This is a key aspect of the opioid management strategy and will also contribute to the growing demand for thebaine derivatives. With the help of biomanufacturing, we can now produce the same thebaine, which was previously only available through poppy farming, in days or weeks, instead of years.
The U.S. government has taken notice of this new opportunity that biomanufacturing presents. U.S. Secretary of State Antony J. Blinken recently highlighted the shortened timeline for Narcan production made possible by these innovations in drug manufacturing. Other government initiatives include the BioMaP-Consortium which is funding Antheia, among other companies, that are leading the adoption of biomanufacturing for more resilient pharmaceutical supply chains in the U.S.
Biomanufacturing has the potential to replace agricultural cultivation of a wide variety of critical pharmaceutical ingredients, offering agile supply chains with consistent quality, predictable yields, and more efficient processes. As a result, drugmakers save time and money, which can drive down the price consumers pay, ultimately improving accessibility.
Thebaine is Antheia’s first biomanufactured KSM product to reach commercial scale and the company is on track to ship its first orders to customers in 2024. As drug manufacturers work to make the FDA’s vision of widely available Narcan a reality, Antheia is leading the effort to transform pharmaceutical supply chains and ensure equitable access to critical, life-saving medicines.
















