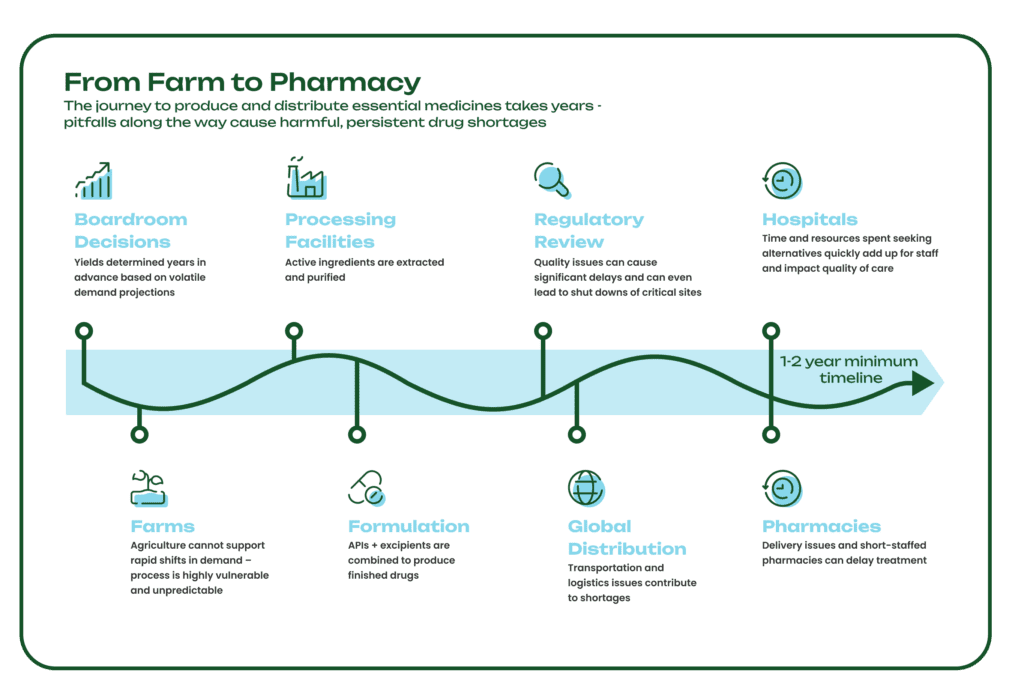
The years-long journey medicines take from the farm to the pharmacy is a delicate one, vulnerable to extreme weather, mechanical failures, logistical and shipping delays, and geopolitical issues. Supply chain disruptions at any point in the process can cause long-lasting drug shortages that have a major impact on patient care. In fact, there are more than 130 drugs currently in shortage, including essential medicines like the antibiotic amoxicillin for bacterial infections, the chemotherapy drug cisplatin, and the anti-seizure medication diazepam.
To understand and address one of the root causes of these shortages, we’ll zoom in on the international process of drug formulation and manufacturing in simple terms. Specifically, we’ll cover how two key components are combined into finished drug products: active pharmaceutical ingredients (APIs) and excipients, often called inactive ingredients since they do not have a pharmacological effect. We’ll also explore the global impact of essential medicine shortages caused by problems in ingredient manufacturing and supply chains.
Understanding active ingredients
Many people are familiar with the term “active ingredients,” which can be found on the labels of over-the-counter and prescription medications like cold medicine, antibiotics, and topical dermatologic products. These APIs are the chemical components of a drug that deliver the intended biological effect. In other words, they are the reason our medicines work. For example, the active ingredient in Tylenol is acetaminophen, a pain reliever and fever reducer. Examples of APIs used to produce other in-demand medications include naloxone hydrochloride for overdose reversal drug Narcan, and scopolamine for postoperative nausea and motion sickness drugs.
Some APIs are produced from Key Starting Materials (KSMs), many of which are sourced directly from nature and specifically, plants (for more on the long history of KSMs, see here). However, plant-derived ingredients are vulnerable to external variables like climate crises, growing seasons, and soil health, which can lead to drug shortages with lasting effects. The agricultural process is also resource-intensive, requiring large amounts of land, water, and chemicals like pesticides. Since it takes several years to grow, harvest, extract, and process medicinal crops, yields and volumes are determined years in advance, and there is no practical way to increase supply when there are shortages or sudden changes in demand.
Without a steady and predictable supply of KSMs and APIs, pharmaceutical supply chains can quickly break down, leaving healthcare systems without the essential medicines they need to treat patients. Physicians face impossible decisions, such as delaying or abandoning treatment plans, and managing shortages can cost hospitals hundreds of thousands of dollars annually.
The pharma industry, healthcare systems, and patients alike need a new mode of drug manufacturing that can support the dynamic needs of a growing global society. By reducing our reliance on costly, inefficient, and unreliable agricultural processes, we can ensure that people have access to the medicines they need.
The role of excipients
APIs are the most critical elements of our finished drug products, but they typically aren’t administered to patients in their native forms. During the drug-making process, manufacturers combine APIs with other materials — excipients — to produce medications in the forms of pills, injections, or topicals. Excipients are listed alongside active ingredients on drug labels and while they don’t have a direct pharmacological effect, they play an important role in stabilizing, administering, accepting, and increasing bioavailability of the API.
Excipients serve many different functions in a drug formulation, including improving drug stability and controlling when and where the active ingredient is released into the body. During the formulation process, drugmakers combine APIs and excipients to meet patients’ dosage and administration needs. Any disruption to the production of these two critical components could delay the rest of the manufacturing process, which in turn impacts the rest of a drug’s journey to hospitals, pharmacies, and doctors’ offices around the world.
In summary, while KSMs and APIs are the linchpins that make drugs work, there must also be a consistent, high-quality supply of common excipients to avoid wider drug shortages.
An international problem
Drug shortages and efforts to counter them are well-documented in countries with high-income economies like the U.S. and throughout the EU, but the problem goes far beyond those borders. In fact, the WHO estimates that over two billion people worldwide lack access to basic medicines. In a study of drug shortages in Colombia from 2010 to 2021, researchers found 173 drugs in shortage, lasting an average of 1.6 years. The longest shortage was for naloxone tablets, a life-saving overdose reversal medication, lasting nearly 10 years. The most common causes of these shortages were manufacturing issues and a small number of suppliers in the market unable to match demand.
Many of these issues stem from the global nature of pharmaceutical supply chains and our widespread dependency on only a few countries for these critical ingredients. Advanced manufacturing technologies, such as synthetic biology and biomanufacturing, can enable efficient, on-demand, and rapid response pharma production that can be globally distributed to diversify and localize where KSMs and APIs are produced. Biomanufacturing has the potential to not only end drug shortages but also bring essential medicines to countries that have historically had little to no access to these life-saving drugs.
Addressing a key root cause
The global and complex nature of the pharmaceutical industry means there are many opportunities for the supply chain to be disrupted. No one company or organization can prevent every problem in this fragile system, but biomanufacturing is the first technology that offers a step change that can truly transform these vital supply chains for essential medicines around the world. We estimate that more than 50 percent of medicines on the U.S. FDA Essential Medicines List can be made with biomanufacturing technology in weeks – rather than years – and at a fraction of the cost of conventional manufacturing approaches.
A 2023 study found that raw material availability and manufacturing issues make up at least 14 percent of shortages. Antheia is directly addressing these issues, replacing legacy agriculture-based processes with biomanufacturing for more resilient, agile, and efficient pharma supply chains.
With cutting edge science, innovative biomanufacturing processes, and strong industry partnerships, we’re building the next generation of global pharmaceutical supply chains and enabling the industry to produce and deliver essential medicines to the people who need them most.

















